Aquarium and pond equipment at the best prices!
For those passionate about aquariophily
Having trouble finalizing your order? Contact us 24 hours a day by email at information@zoanthus.fr.
Basin Analysis
5€ discount for your first order with the code: BIENVENUE05 (from 100€ purchase, offers cannot be combined)
Balling Products
Seawater supplements
Basic elements
Fresh Water Supplements
CO2 system
5€ discount for your first order with the code: BIENVENUE05 (from 100€ purchase, offers cannot be combined)
Water treatment
Bacterial Treatments
Adverse Treatments
Fish Care
Water treatment
Bacterial Treatments
Adverse Treatments
Fish Care
Water treatment
Bacterial Treatments
Fish Care
5€ discount for your first order with the code: BIENVENUE05 (from 100€ purchase, offers cannot be combined)
5€ discount for your first order with the code: BIENVENUE05 (from 100€ purchase, offers cannot be combined)
Sand
Substrate for reactor
Sea Salts
Pond Substrate
5€ discount for your first order with the code: BIENVENUE05 (from 100€ purchase, offers cannot be combined)
Seawater Equipment
Basin Equipment
5€ discount for your first order with the code: BIENVENUE05 (from 100€ purchase, offers cannot be combined)


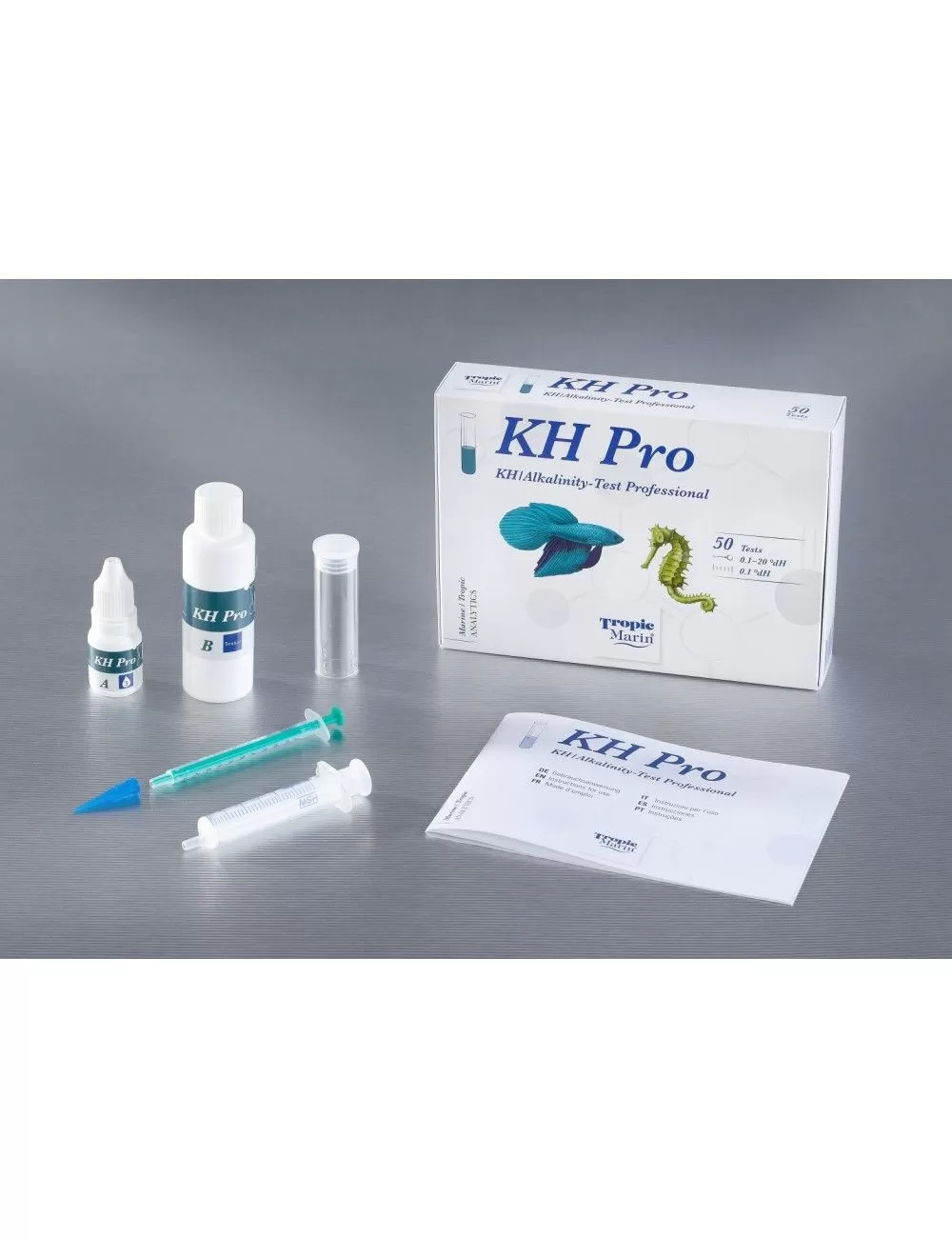
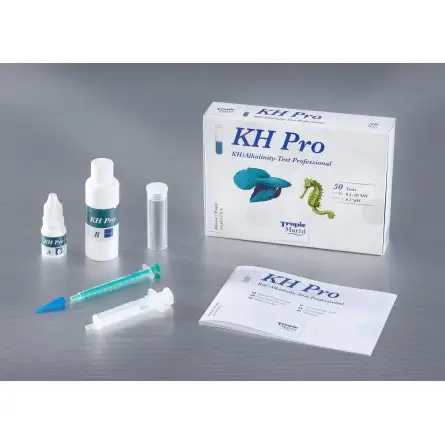
Refill reagents for the Tropic Marin Professional KH Test .
Non contractual photo.
You may also like
The Tropic Marin ® Professional KH Test is a highly accurate test kit for the determination of alkalinity with a resolution of up to 0.1 °dH per titration including a conversion table to other units.
| • | Measuring range: 0.1 – 20 °dH |
| • | Accuracy: 0.1°dH |
| • | For highly accurate determination of carbonate hardness in aquaria |
| • | Sufficient for approx. 100 applications at 10°dH KH in water and 0.2°dH resolution / or 50 applications at 0.1°dH resolution |
| • | The indication is given in degrees of German hardness (°dH) – conversion table for other units in the included instructions |
For highly accurate determination of carbonate hardness (KH)
The carbonate hardness (KH) or alkalinity of a water sample refers to the buffer capacity, i.e. the ability to maintain the pH value of the water. In water chemistry, various terms describe buffering capacity with different definitions. In aquariums, the term "carbonate hardness" is common; the measurement relates to the alkalinity. In this test, the two terms are synonymous. As a rule, carbonate hardness is expressed in degrees of German hardness (°dH).
The carbonate hardness must be checked very regularly in all aquariums. Low alkalinity in the aquarium can lead to a drop in pH value that is fatal for many fish and invertebrates. In coral aquariums, the carbonate hardness must be sufficient to ensure vigorous growth of corals. On the other hand, too high a carbonate hardness in a saltwater pool can lead to lime deposits and harm the growth of corals. In a saltwater aquarium, the carbonate hardness should be between 6 and 9 °dH. In freshwater aquaria the carbonate hardness should not be less than 3 °dH.
| 1. | Before use, shake the bottles well! |
| 2. | Rinse the glass bowl several times with tap water, then with aquarium water. |
| 3. | Using the dosing syringe, precisely pour 5 ml of aquarium water into the glass bowl. |
| 4. | Then add 3 drops of reagent A (indicator) and carefully swirl the cuvette. The water sample takes on a turquoise color. |
| 5. | Place the tip provided on the small syringe and draw up 1 ml of reagent B (titrator). |
| 6. | Now add reagent B from the syringe drop by drop into the water sample until its color changes from turquoise to light pink to dark blue and purple. After each drop, carefully swirl the cuvette. The measurement is complete when the color of the sample has reached a light pink tone without a bluish tint. |
| 7. | The consumption of reagent B (difference compared to 1 ml) multiplied by 10 makes it possible to obtain the carbonate hardness in °dH. Example: If the lower end of the syringe plunger is after the end of the titration at 0.28 ml, the consumption of reagent B is 0.72 ml (difference from 1 ml). 0.72 x 10 = 7.2. The carbonate hardness of aquarium water is 7.2 °dH. For a wider range of test reagents or in the case of hard water above 10°dH, the test can be performed with a 2.5 ml sample. Proceed as described previously and, at the end of the titration, multiply the consumption of reagent B by 20. |
| 8. | After the measurement process is complete, rinse the glass cuvette, syringe and syringe tip thoroughly with tap water. |
| Analysis |
|
Anti-silicate filter with a capacity of 500 ml. This filter is intended to remove silicates and other components from water that cannot be 100% removed by reverse osmosis. It is to be placed at the osmosis outlet.
Scope of delivery: housing, fittings, wall bracket, 1000ml resin.
JBl's ArtemioFluid is a liquid food for the rearing of live food such as brine shrimp. It is based on homogenized phytoplankton and other essential nutrients for feeding Artemia nauplii.
This SALIFERT brand analysis kit allows you to accurately measure the calcium level of your seawater. Allows you to take 50 to 100 measurements.
CUPRAMINE™ is effective in the eradication of ectoparasites from freshwater and saltwater fish.
The third element of the Balling method, this "salt-free salt" is a mixture of all the mineral salts present in natural seawater , except sodium chloride NaCl. It allows the maintenance of the ionic balance in the application of the Balling method.
1kg of powder allows the preparation of 41 liters of solution. That's 0€41 per litre!
High purity chemical products compliant with the European Pharmacopoeia prepared and packaged on site in our warehouse.
Products are now packaged in resealable pouches for less breakage during transport. They are also more ecological with 80% less plastic compared to our old packaging.
SEACHEM Pristine is a 100% natural product composed of a unique blend of specific bacteria that improve water quality in freshwater and saltwater aquariums. These bacteria have been selected for their robustness and their ability to survive. adapt to all aquatic environments.
Anti-silicate filter with a capacity of 600 ml for demineralization resin. This filter is intended to remove silicates and other components from water that cannot be 100% removed by reverse osmosis. It is to be placed at the osmosis outlet.
Scope of delivery: housing, fittings, wall bracket, 600ml resin.
Anti-silicate filter with a capacity of 500 ml. This filter is intended to remove silicates and other components from water that cannot be 100% removed by reverse osmosis. It is to be placed at the osmosis outlet.
Scope of delivery: housing, fittings, wall bracket, 1000ml resin.
JBl's ArtemioFluid is a liquid food for the rearing of live food such as brine shrimp. It is based on homogenized phytoplankton and other essential nutrients for feeding Artemia nauplii.
This SALIFERT brand analysis kit allows you to accurately measure the calcium level of your seawater. Allows you to take 50 to 100 measurements.
CUPRAMINE™ is effective in the eradication of ectoparasites from freshwater and saltwater fish.
The third element of the Balling method, this "salt-free salt" is a mixture of all the mineral salts present in natural seawater , except sodium chloride NaCl. It allows the maintenance of the ionic balance in the application of the Balling method.
1kg of powder allows the preparation of 41 liters of solution. That's 0€41 per litre!
High purity chemical products compliant with the European Pharmacopoeia prepared and packaged on site in our warehouse.
Products are now packaged in resealable pouches for less breakage during transport. They are also more ecological with 80% less plastic compared to our old packaging.
SEACHEM Pristine is a 100% natural product composed of a unique blend of specific bacteria that improve water quality in freshwater and saltwater aquariums. These bacteria have been selected for their robustness and their ability to survive. adapt to all aquatic environments.
Anti-silicate filter with a capacity of 600 ml for demineralization resin. This filter is intended to remove silicates and other components from water that cannot be 100% removed by reverse osmosis. It is to be placed at the osmosis outlet.
Scope of delivery: housing, fittings, wall bracket, 600ml resin.
Anti-silicate filter with a capacity of 500 ml. This filter is intended to remove silicates and other components from water that cannot be 100% removed by reverse osmosis. It is to be placed at the osmosis outlet.
Scope of delivery: housing, fittings, wall bracket, 1000ml resin.
JBl's ArtemioFluid is a liquid food for the rearing of live food such as brine shrimp. It is based on homogenized phytoplankton and other essential nutrients for feeding Artemia nauplii.
This SALIFERT brand analysis kit allows you to accurately measure the calcium level of your seawater. Allows you to take 50 to 100 measurements.
With the Professional Phosphate Test from Tropic Marin ® the phosphate concentration can be determined with very high accuracy in the low concentration range between 0.01 and 1 mg/l. Allows you to take around 50 measurements.
Refill reagents for the Phosphat PO4 Professional test from Tropic Marin .
Non contractual photo.
This Professional Potassium Test from Tropic Marin ® reliably determines the potassium concentration with a resolution of 5 mg/l.
The Tropic Marin ® Professional KH Test is a highly accurate test kit for the determination of alkalinity with a resolution of up to 0.1 °dH per titration including a conversion table to other units.
With the Professional Nitrite/Nitrate Test from Tropic Marin ® the concentration of nitrites and nitrates can be determined with very high accuracy in the low concentration range.
This Professional Combined Calcium/Magnesium Test from Tropic Marin ® determines the concentration of each of these elements reliably and separately with very high precision.
The Tropic Marin ® Tropic Marin Test allows the control of the pH value thanks to a precise palette of colors (pH ranging from 7.4 to 9.4).
With the Professional Phosphate Test from Tropic Marin ® the phosphate concentration can be determined with very high accuracy in the low concentration range between 0.01 and 1 mg/l. Allows you to take around 50 measurements.
Refill reagents for the Phosphat PO4 Professional test from Tropic Marin .
Non contractual photo.
This Professional Potassium Test from Tropic Marin ® reliably determines the potassium concentration with a resolution of 5 mg/l.
The Tropic Marin ® Professional KH Test is a highly accurate test kit for the determination of alkalinity with a resolution of up to 0.1 °dH per titration including a conversion table to other units.
With the Professional Nitrite/Nitrate Test from Tropic Marin ® the concentration of nitrites and nitrates can be determined with very high accuracy in the low concentration range.
This Professional Combined Calcium/Magnesium Test from Tropic Marin ® determines the concentration of each of these elements reliably and separately with very high precision.
The Tropic Marin ® Tropic Marin Test allows the control of the pH value thanks to a precise palette of colors (pH ranging from 7.4 to 9.4).
With the Professional Phosphate Test from Tropic Marin ® the phosphate concentration can be determined with very high accuracy in the low concentration range between 0.01 and 1 mg/l. Allows you to take around 50 measurements.
Refill reagents for the Phosphat PO4 Professional test from Tropic Marin .
Non contractual photo.
This Professional Potassium Test from Tropic Marin ® reliably determines the potassium concentration with a resolution of 5 mg/l.
What they think about it
(and they are right...)
 Alessandro D.
Alessandro D.

Absolutely valifo product (Translated review)